Graphical abstract. Credit: The Journal of Physical Chemistry C (2023). DOI: 10.1021/acs.jpcc.3c05676
Chemists at the Department of Energy’s Oak Ridge National Laboratory have invented a more efficient way to extract lithium from waste liquids leached from mining sites, oil fields, and used batteries. They demonstrated that a common mineral can adsorb at least five times more lithium than can be collected using previously developed adsorbent materials.
“It’s a low-cost, high-lithium-uptake process,” said Parans Paranthaman, an ORNL Corporate Fellow and National Academy of Inventors Fellow with 58 issued patents. He led the proof-of-concept experiment with Jayanthi Kumar, an ORNL materials chemist with expertise in the design, synthesis, and characterization of layered materials.
“The key advantage is that it works in a wider pH range of 5 to 11 compared to other direct lithium extraction methods,” Paranthaman said. The acid-free extraction process takes place at 140 degrees Celsius, compared to traditional methods that roast mined minerals at 250 degrees Celsius with acid or 800 to 1000 degrees Celsius without acid.
The team has applied for a patent for the invention.
Lithium is a lightweight metal commonly used in energy-dense and rechargeable batteries. Electric vehicles, which are needed to achieve net-zero emissions by 2050, rely on lithium-ion batteries. Industrially, lithium is extracted from brines, rocks and clays. The ORNL innovation may help meet rising demand for lithium by making domestic sources commercially viable.
The research reveals a route away from the status quo: a linear economy in which materials from mining, refining or recycling are made into products that, at the end of their lives, are discarded as waste. The work moves toward a circular economy in which materials are kept in circulation as long as possible to reduce the consumption of virgin resources and the generation of waste.
To support a circular economy, aluminum hydroxide can extract 37 milligrams of lithium per gram of recoverable sorbent in a single step. Credit: Jayanthi Kumar, Parans Paranthaman and Philip Gray/ORNL, U.S. Dept. of Energy
The ORNL invention relies on aluminum hydroxide, a mineral that is abundant in Earth’s crust. The scientists used aluminum hydroxide as a sorbent, which is a material that takes up another material—in this case, lithium sulfate—and holds it.
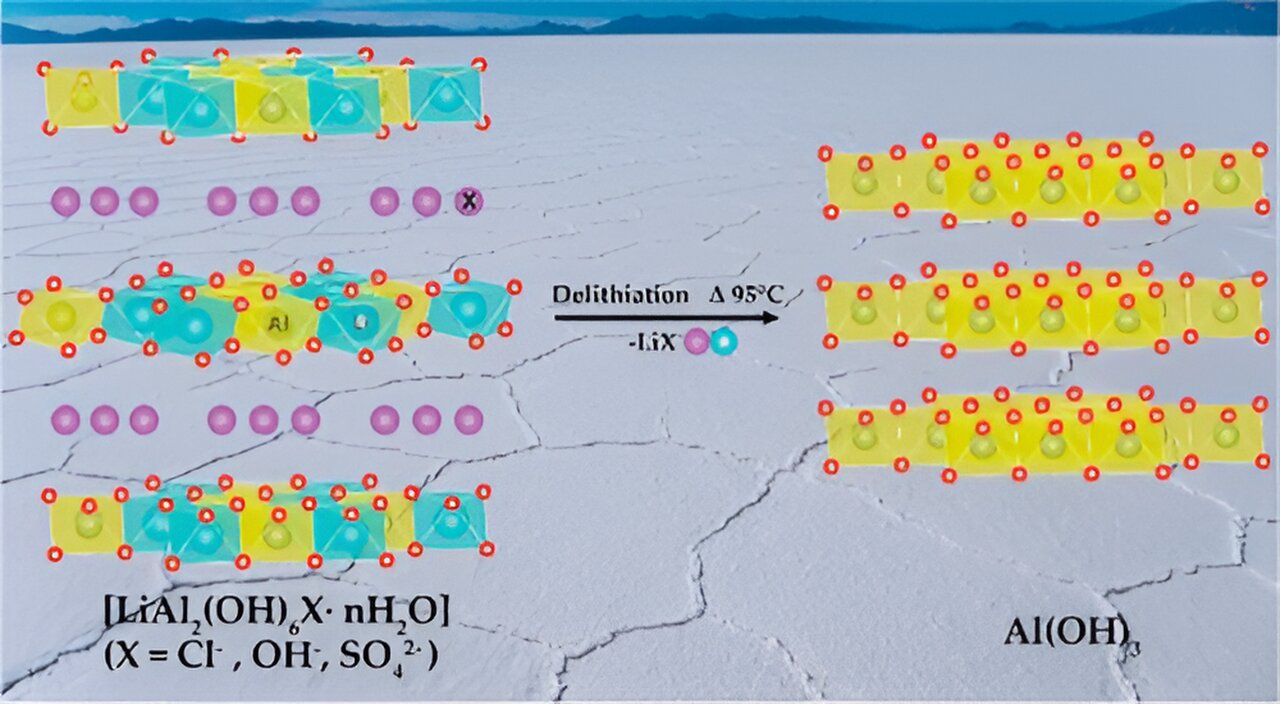
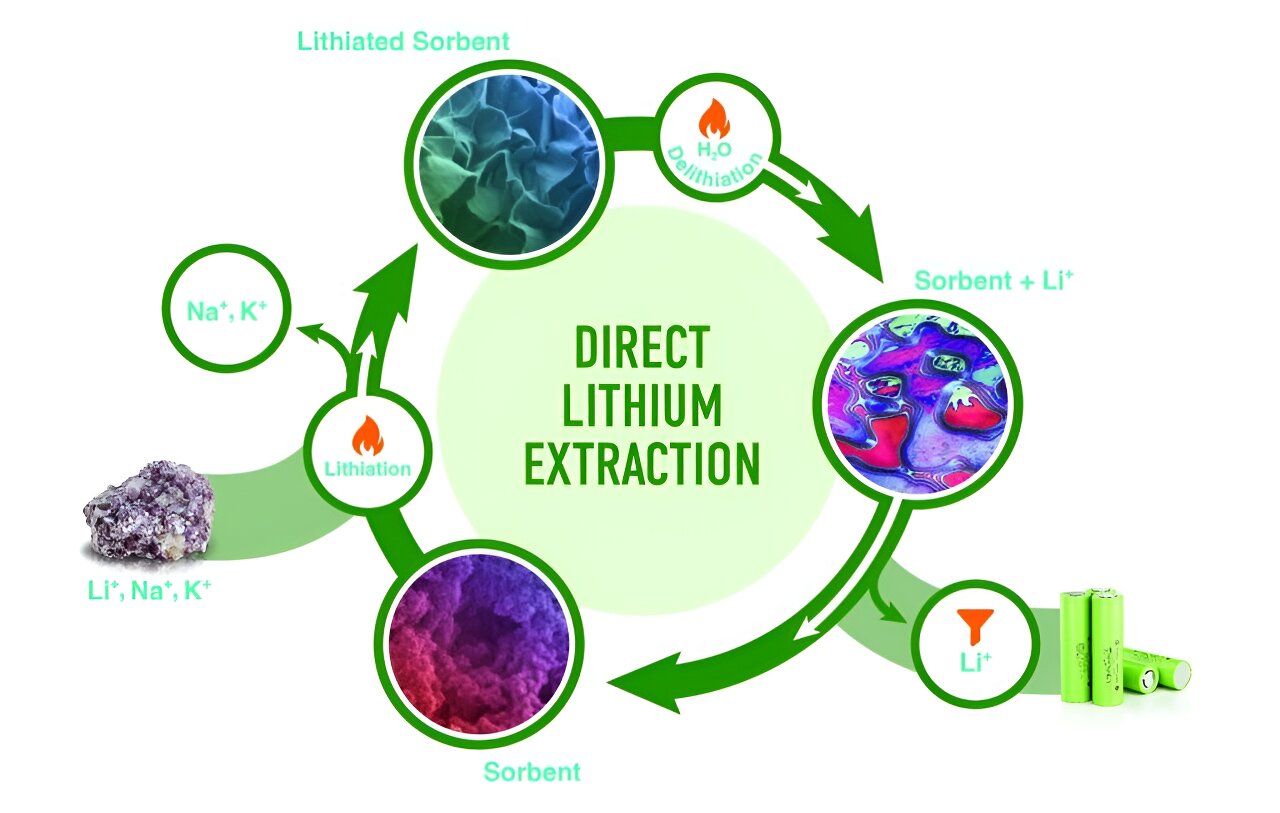
In a process called lithiation, an aluminum hydroxide powder extracts lithium ions from a solvent to form a stable layered double hydroxide, or LDH, phase. Then in delithiation, treatment with hot water causes the LDH to relinquish lithium ions and regenerate the sorbent. During relithiation, the sorbent is reused to extract more lithium. “This is the basis for a circular economy,” Paranthaman said.
The research is published in the journal ACS Applied Materials & Interfaces. A related second paper, concurrently published in The Journal of Physical Chemistry C, explored the stability of delithiation under various conditions.
Aluminum hydroxide exists in four highly ordered crystalline polymorphs and one amorphous, or disordered, form. Form turns out to play a big role in the sorbent’s function.
Kumar traveled to Arizona State University to work with Alexandra Navrotsky to measure thermodynamics of chemical reactions. ORNL Corporate Fellow Bruce Moyer, a renowned expert in separation science and technology, provided insight into the kinetic experiments.
“Based on calorimetric measurements, we learned that amorphous aluminum hydroxide is the least stable form among aluminum hydroxides and thus is highly reactive,” said Kumar. “That was a key to this method resulting in greater lithium extraction capacity.”
Because amorphous aluminum hydroxide is the least stable among the mineral’s forms, it spontaneously reacts with lithium from brine leached from waste clays. “Only when we did the measurements did we realize that the amorphous form is way, way, way less stable. That is why it is more reactive,” Kumar said. “To gain stability, it reacts very quickly compared to other forms.”
Kumar is optimizing the process by which the sorbent selectively adsorbs lithium from liquids containing lithium, sodium and potassium and goes on to form LDH sulfate.
At the Center for Nanophase Materials Sciences, a DOE Office of Science user facility at ORNL, the researchers used scanning electron microscopy to characterize the morphology of aluminum hydroxide during lithiation. It is a charged neutral layer that contains atomic vacancies, or tiny holes. Lithium is absorbed at these sites. The size of these vacancies is the key to aluminum hydroxide’s selectivity for lithium, which is a positively charged ion, or cation.
“That vacant site is so small that it can fit only cations the size of lithium,” Kumar said. “Sodium and potassium are cations with larger radii. The bigger cations don’t fit into the vacant site. However, it’s a perfect match for lithium.”
The selectivity of amorphous aluminum hydroxide for lithium results in near-perfect efficiency. In a single step, the process captured 37 milligrams of lithium per gram of recoverable sorbent—approximately five times more than a crystalline form of aluminum hydroxide called gibbsite, which was previously employed for lithium extraction.
The first step of lithiation extracts 86% of the lithium in the leachate, or brine, from mining sites or oil fields. Running the leachate through the amorphous aluminum hydroxide sorbent a second time picks up the rest of the lithium. “In two steps, you can fully recover the lithium,” Paranthaman said.
Venkat Roy and Fu Zhao at Purdue University analyzed life cycle benefits of a circular economy from direct lithium extraction. They compared the ORNL process to a standard method using sodium carbonate. They found the ORNL technology used one-third the material and one-third the energy and subsequently generated fewer greenhouse gas emissions.
Next, the researchers want to extend the process to extract more lithium and regenerate the sorbent in a specific form. Now, when the amorphous aluminum hydroxide sorbent reacts with the lithium and is later treated with hot water to remove the lithium and regenerate the sorbent, the result is a structural change in the aluminum hydroxide polymorph from amorphous to a crystalline form called bayerite.
“The bayerite form is less reactive,” Kumar said. “It requires either more time—18 hours—or more concentrated lithium for it to react, as opposed to the amorphous form, which reacts within 3 hours to pick all the lithium from the leachate solution. We need to find a route to get back to the amorphous phase, which we know is highly reactive.”
Success in optimizing the new process for extraction speed and efficiency could be a game-changer for the domestic lithium supply. More than half of the world’s land-based lithium reserves are in places where the concentration of dissolved minerals is high, such as California’s Salton Sea or oil fields in Texas and Pennsylvania.
“Domestically, we don’t really have lithium manufacturing,” Paranthaman said. “Less than 2% of lithium for manufacturing is from North America. If we can use the new ORNL process, we have various lithium sources all over the United States. The sorbent is so good you can use it for any brines or even solutions from recycled lithium-ion batteries.”
More information: K. Jayanthi et al, Integrated Circular Economy Model System for Direct Lithium Extraction: From Minerals to Batteries Utilizing Aluminum Hydroxide, ACS Applied Materials & Interfaces (2023). DOI: 10.1021/acsami.3c12070
K. Jayanthi et al, Effect of Anions on the Delithiation of [Li–Al] Layered Double Hydroxides: Thermodynamic Insights, The Journal of Physical Chemistry C (2023). DOI: 10.1021/acs.jpcc.3c05676
Provided by Oak Ridge National Laboratory
This story was originally published on Phys.org. Subscribe to our newsletter for the latest sci-tech news updates.
News Related-
Russian court extends detention of Wall Street Journal reporter Gershkovich until end of January
-
Russian court extends detention of Wall Street Journal reporter Evan Gershkovich, arrested on espionage charges
-
Israel's economy recovered from previous wars with Hamas, but this one might go longer, hit harder
-
Stock market today: Asian shares mixed ahead of US consumer confidence and price data
-
EXCLUSIVE: ‘Sister Wives' star Christine Brown says her kids' happy marriages inspired her leave Kody Brown
-
NBA fans roast Clippers for losing to Nuggets without Jokic, Murray, Gordon
-
Panthers-Senators brawl ends in 10-minute penalty for all players on ice
-
CNBC Daily Open: Is record Black Friday sales spike a false dawn?
-
Freed Israeli hostage describes deteriorating conditions while being held by Hamas
-
High stakes and glitz mark the vote in Paris for the 2030 World Expo host
-
Biden’s unworkable nursing rule will harm seniors
-
Jalen Hurts: We did what we needed to do when it mattered the most
-
LeBron James takes NBA all-time minutes lead in career-worst loss
-
Vikings' Kevin O'Connell to evaluate Josh Dobbs, path forward at QB
